NDMA Found in Ranitidine
Written by Bryan Tan – Marketing and Communications Associate at YPC, currently a PRP at a retail pharmacy, and pun enthusiast
Recently, there has been news regarding the discovery of traces of N-Nitrosodimethylamin (NDMA) found in ranitidine medications. This was reported by the American-based Food and Drug Administration (FDA) as well as the Singaporean Health Science Authority (HSA). Only the HSA has recalled ranitidine products so far, with the FDA and also European Medicines Agency (EMA) still monitoring the situation.
So…what’s all the fuss about?
This is not the first time this has happened, with the commotion about recalls of losartan medications (an antihypertensive) just months before with the exact same reason as this time – traces of NDMA found.
What’s the big deal about NDMA, you ask? NDMA is classified as a probable carcinogen (that is, has the potential to cause cancer). That’s right, CANCER.
So why hasn’t the Malaysian government recalled these products? Why wait for it to cause more harm? Well, according to the FDA, NDMA is a known environmental contaminant and is found in water and foods. They only cause harm at high levels and the FDA is finding that the level of NDMA found in these samples is as low as those that you find in common foods.
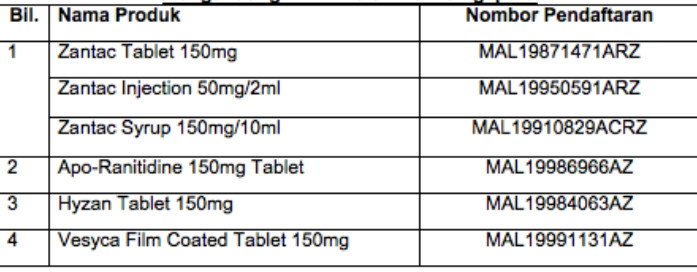
The HSA, however, has a different approach as they have found these traces to be above internationally accepted levels, hence the recalls. These recalls are applicable to these 8 particular brands:
| PRODUCT NAME | LOCAL SUPPLIER | |
| 1 | Aciloc 150 Tablet 150 mg
Aciloc 300 Tablet 300 mg |
Uni Drug House |
| 2 | Apo-Ranitidine Tablet 150 mg | Pharmaforte Singapore Pte Ltd |
| 3 | Hyzan Tablet 150 mg | Apex Pharma Marketing Pte Ltd |
| 4 | Neoceptin R-150 Tablet 150 mg | Pharmatech Resources (FE) Pte Ltd |
| 5 | Vesyca Film Coated Tablet 150 mg | Yung Shin Pharmaceutical (Singapore) Pte Ltd |
| 6 | Xanidine Tablet 150 mg | Polymedic Trading Enterprise Pte Ltd |
| 7 | Zantac Injection 25 mg/ml
Zantac Syrup 150 mg/10 ml Zantac Tablet 150 mg |
GlaxoSmithKline Pte Ltd |
| 8 | Zynol-150 Tablet 150 mg | Naina Mohamed & Sons Private Limited |
Only 4 out of these 8 brands are currently registered in Malaysia, and they are Apo-ranitidine, Hyzan, Vesyca, and Zantac.
But..what IS ranitidine?
If you are not familiar with this medication, it is a H2 receptor antagonist which functions to decrease the amount of acid created by the stomach.
If you have friends or family who take these brands and are concerned, here is what you should tell them: DO NOT STOP TAKING IT ON THEIR OWN. Consult their nearest pharmacist or doctor if they are worried. It is not safe to stop taking medications without the advice of a healthcare professional as there are times when the benefit outweighs the risk.
Our health ministry has said that they will be monitoring this closely and will keep the public updated. We too will be doing the same, so until then, stay tuned!