Macrolide: ‘BANG” on the Rhythm

Written by J. Z. Low – Clinical pharmacist, and a photography enthusiast.
Macrolides are one of the antibiotics that are widely prescribed for ranges of infectious diseases including upper and lower respiratory tract diseases, sexually transmitted diseases and certain skin infections caused by zoonotic origins.
The widespread use of macrolides rises a concern in their adverse effect, mainly in cardiotoxicity. Much studies reported macrolides are strongly related to cardiotoxicity [1-3]. The cardio-related adverse events of macrolides often reported as QT interval prolongation, torsades de pointes (TdP), ventricular arrhythmia and sudden cardiac arrest [4, 5]. A great variety and distinctive ion transporters and ion channels exit on the myocyte which regulate the cardiac inotropy and chronotropy. Macrolides inhibit of the delayed rectifier potassium channels (IKr), which regulate the outflow of potassium ions from the myocytes[6]. As a result of impaired functioning of these potassium channels, potassium ions accumulate excessively inside the myocytes which eventually delay the ventricular repolarization. Ultimately, the delay in ventricular repolarization is measured by the prolongation in QT interval.
The purpose of this review is to highlight the core findings of hallmark studies of macrolide associated cardiotoxicity.
Erythromycin
Erythromycin is the first agent discovered in the macrolide class of antibiotics. A comprehensive review performed by Tschida et al. which reviewed 17 cases reports and included 23 patients that found the use of infusion erythromycin lactobionate in critically-ill patients associated with the development of QT interval prolongation and TdP [7]. However, fourteen patients in the studies had underlying cardiac diseases. In addition, the remaining patients had risk factors contributing to the developments of cardio-arrhythmia which were, rapid infusion of erythromycin lactobionate, active liver disease and medications which are known to cause QT prolongation.
Azithromycin
Previously, azithromycin was known to be less associated with cardio-arrhythmia toxicity [6]. However, there were growing evidence reporting that patients with normal baseline echocardiogram (ECG) developed arrythmia-related adverse cardiac events such as TdP [8], QT interval prolongation [9, 10] and polymorphic ventricular tachycardia without presence of QT interval prolongation [11].
A large retrospective cohort which included more than 300,000 prescriptions of azithromycin done in US to assess the cardiovascular death associated with azithromycin reported that a short course of 5 days azithromycin therapy increased cardiovascular-related death and all-cause mortality by 188% and 85% respectively when compared to propensity-matched controls who did not receive any antibiotics [12]. Moreover, patients with azithromycin treatment when compared to patient with amoxicillin therapy, the former had double the risk of suffering from cardiovascular death and all-cause mortality. Although the author had taken the necessary measures to minimize the confounding factors in the study, but the risk appeared to be higher among patients with known heart diseases and severity of underlying diseases.
As a result of this study, Food Drug Administration (FDA) on 2013 released a warning statement regarding patients who filled azithromycin prescription had a higher risk of developing QT interval prolongation and potential fatal TdP.
Clarithromycin
The CLARICOR trial was a randomized, placebo controlled and multicenter trial which studied the cardiotoxicity of clarithromycin in patients with stable coronary hearth diseases for the treatment of atherosclerosis [13]. This study included over 4000 patients with 10-years of follow up. The trial found that clarithromycin increased the overall all-cause mortality by 10%. During the first 3 years of follow up, the cardiovascular mortality increased by 42% in patients treated with clarithromycin when compared to placebo. In addition, the study also reported that mortality from cerebrovascular diseases was higher among patients taking clarithromycin. Furthermore, few cohort studies also found that clarithromycin increased the mortality of cardiovascular origins [2, 3].
Although the aforementioned studies found that macrolides are strongly associated with cardiovascular toxicity, large cohort studies which included more than 600,000 patients received treatment with macrolides versus those without macrolide treatment (amoxicillin, cefuroxime or levofloxacin) produced a differing result [14]. The researchers matched over 100 baseline characteristics between the macrolide group and non-macrolide group to minimize the confounding in the study. The study did not find risk of 30 days ventricular arrythmia higher in the macrolide group when compared to the non-macrolide arm. Surprisingly, the non-macrolide arm had a lower all-cause mortality rate when compared to the macrolide group [relative risk, 95% confidence interval: 0.82 (0.78–0.86)]. Subgroup analysis also revealed that patients with chronic kidney disease, congestive heart failure, coronary artery disease and concomitant taking QT-prolonging drugs did not pose additional risk of developing 30-days ventricular arrhythmia. A systematic review which included thirty-three studies to investigate the link between macrolides and cardiac-associated adverse events did not find macrolides increased all-cause mortality [4]. The most recent systematic review and meta-analysis also showed that treatment with macrolides did not increase risk of arrhythmia or cardiovascular mortality [15].
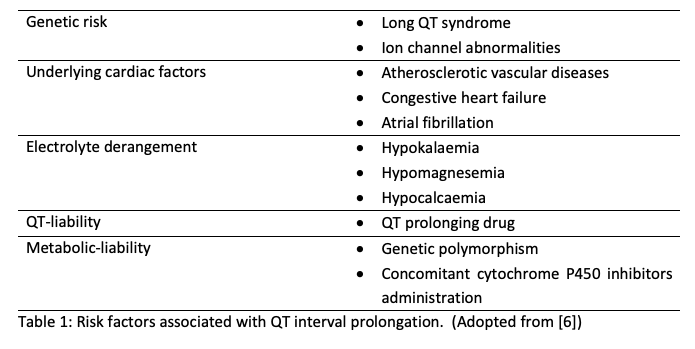
The risks of developing macrolide associated cardio-arrhythmia are often affected by two major factors which are ‘QT liability’ of macrolides which affects the normal functioning of IKr, and ‘metabolic liability’ which involves the metabolism of macrolide by cytochrome P450 [6]. Concomitant administration of cytochrome P450 inhibitors such as anti-histamines and antifungals can increase myocytes exposure to the IKr-inhibiting agents which may result in a surge of developing cardio-arrhythmia. In addition, exposure to macrolide and underlying comorbidities amplify the probability of causing cardio-arrhythmia in patients (Table 1). All these factors will exhaust the repolarization reserve in the ventricle and result in electrical instability in the membrane of myocytes which ultimately cause arrhythmia.
Published literature had shown conflicting result on risk of developing cardiotoxicity during treatment with macrolides. Although recent systemic review [15] and cohort studies [14] reassured the cardio-safety of macrolides, but these results should be interpreted with caution especially in high risk patients. Most of the patients presented to health care are complicated with multiple comorbidities. The unpredicted pharmacokinetics and pharmacodynamics parameters in these kinds of patients, together with underlying abnormal electrical physiology of heart will pose a greater risk of developing arrhythmia. A baseline electrocardiogram should be done in all patients before starting macrolide to ensure there is no underlying pathology which can increase the risk of cardiotoxicity when treating patients.
References:
1. Milberg P, Eckardt L, Bruns HJ, Biertz J, Ramtin S, Reinsch N, et al. Divergent proarrhythmic potential of macrolide antibiotics despite similar QT prolongation: fast phase 3 repolarization prevents early afterdepolarizations and torsade de pointes. The Journal of pharmacology and experimental therapeutics. 2002;303(1):218-25.
2. Schembri S, Williamson PA, Short PM, Singanayagam A, Akram A, Taylor J, et al. Cardiovascular events after clarithromycin use in lower respiratory tract infections: analysis of two prospective cohort studies. BMJ (Clinical research ed). 2013;346:f1235.
3. Svanström H, Pasternak B, Hviid A. Use of clarithromycin and roxithromycin and risk of cardiac death: cohort study. BMJ : British Medical Journal. 2014;349:g4930.
4. Cheng YJ, Nie XY, Chen XM, Lin XX, Tang K, Zeng WT, et al. The Role of Macrolide Antibiotics in Increasing Cardiovascular Risk. Journal of the American College of Cardiology. 2015;66(20):2173-84.
5. Abo-Salem E, Fowler JC, Attari M, Cox CD, Perez-Verdia A, Panikkath R, et al. Antibiotic-induced cardiac arrhythmias. Cardiovascular therapeutics. 2014;32(1):19-25.
6. Owens RC, Jr., Nolin TD. Antimicrobial-associated QT interval prolongation: pointes of interest. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2006;43(12):1603-11.
7. Tschida SJ, Guay DRP, Straka RJ, Hoey LL, Johanning R, Vance-Bryan K. QTc-Interval Prolongation Associated with Slow Intravenous Erythromycin Lactobionate Infusions in Critically Ill Patients: A Prospective Evaluation and Review of the Literature. Pharmacotherapy: The Journal of Human Pharmacology and Drug Therapy. 1996;16(4):663-74.
8. Kezerashvili A, Khattak H, Barsky A, Nazari R, Fisher JD. Azithromycin as a cause of QT-interval prolongation and torsade de pointes in the absence of other known precipitating factors. Journal of interventional cardiac electrophysiology : an international journal of arrhythmias and pacing. 2007;18(3):243-6.
9. Russo V, Puzio G, Siniscalchi N. Azithromycin-induced QT prolongation in elderly patient. Acta bio-medica : Atenei Parmensis. 2006;77(1):30-2.
10. Samarendra P, Kumari S, Evans SJ, Sacchi TJ, Navarro V. QT prolongation associated with azithromycin/amiodarone combination. Pacing and clinical electrophysiology : PACE. 2001;24(10):1572-4.
11. Kim MH, Berkowitz C, Trohman RG. Polymorphic ventricular tachycardia with a normal QT interval following azithromycin. Pacing and clinical electrophysiology : PACE. 2005;28(11):1221-2.
12. Ray WA, Murray KT, Hall K, Arbogast PG, Stein CM. Azithromycin and the Risk of Cardiovascular Death. New England Journal of Medicine. 2012;366(20):1881-90.
13. Winkel P, Hilden J, Hansen JF, Kastrup J, Kolmos HJ, Kjoller E, et al. Clarithromycin for stable coronary heart disease increases all-cause and cardiovascular mortality and cerebrovascular morbidity over 10years in the CLARICOR randomised, blinded clinical trial. International journal of cardiology. 2015;182:459-65.
14. Trac MH, McArthur E, Jandoc R, Dixon SN, Nash DM, Hackam DG, et al. Macrolide antibiotics and the risk of ventricular arrhythmia in older adults. CMAJ : Canadian Medical Association journal = journal de l’Association medicale canadienne. 2016;188(7):E120-e9.
15. Gorelik E, Masarwa R, Perlman A, Rotshild V, Muszkat M, Matok I. Systematic Review, Meta-analysis, and Network Meta-analysis of the Cardiovascular Safety of Macrolides. Antimicrobial agents and chemotherapy. 2018;62(6).
The opinions expressed in the article are the writer’s own and do not reflect the view of MPS YPC.

Low Joo Zheng is a clinical pharmacist in Hospital Kuala Krai, Kelantan. He is a registered pharmacist with a deep interest in anti-microbial and cardiology. He actively participates in the Anti-microbial Stewardship team in the hospital. He is often invited to share his taught regarding judicious use of anti-microbial agents during district and hospital level seminars. He is the member of anti-microbial auditing team which helps to monitor the usage of board spectrum antibiotics in the hospital. He is also actively engaged in research, especially in anti-microbial and secondary prevention of atherosclerosis cardiovascular disease.