PRP FAQ Series: Pharmaceutical Industry

In this series, ex or current Provisionally Registered Pharmacists (PRPs) will share their experiences in respective industries across the pharmacy landscape in Malaysia. This article will be focusing on PRP-ship in the pharmaceutical industry.
Thinking of embarking on the path less taken?
While liberalisation of PRP-ship took flight in October 2012, the number of PRPs in the pharmaceutical industry are only a scarce few compared to those who entered the government sector.
This can be attributed to multiple factors, i.e. limited openings, preference of clinical setting, peer influence.
If you are still unsure whether or not to do your PRP-ship in the pharmaceutical industry, or you have decided on this path but are not sure where to begin, we hope the FAQ and tips below will be of guidance to you.
The Basics
1. What is the pharmaceutical industry like in Malaysia?
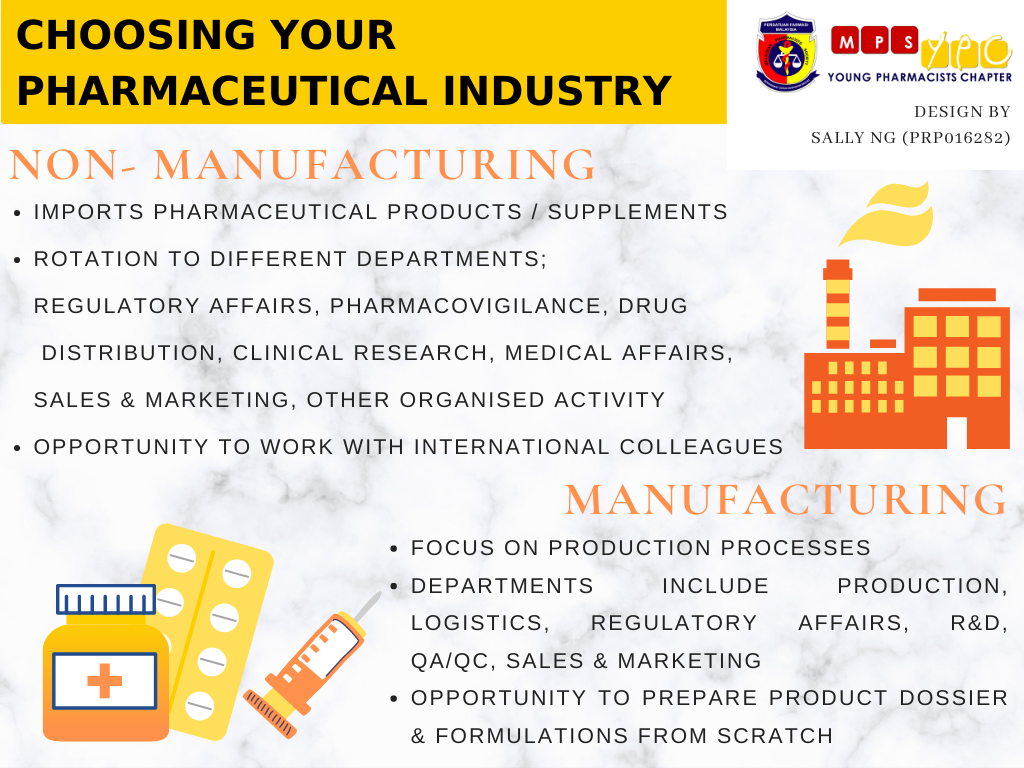
The pharmaceutical industry in Malaysia can generally be categorized into manufacturing and non-manufacturing pharmaceutical industries; both of which are viable options to undergo your PRP-ship.
The manufacturing pharmaceutical industry largely comprises pharmaceutical companies that manufacture pharmaceutical products locally, while the non-manufacturing pharmaceutical industry is generally made up of multinational companies that import and market pharmaceutical products locally.
2. Can I apply to all pharmaceutical companies in Malaysia?
The list of pharmaceutical companies that are acknowledged by the Pharmacy Board of Malaysia as PRP training premises can be found on the Pharmaceutical Services Programme website.
Do note that the intake of PRPs and the number of PRPs per intake varies between companies and is typically dependent on the vacancy at the time.
The Application

Non-manufacturing pharmaceutical industry:
Despite its name, the pharmaceutical companies that fall under this category do manufacture pharmaceutical products, albeit not in Malaysia. They largely import pharmaceutical products and supplements from GMP-certified manufacturers in other countries that have to be registered with the National Pharmaceutical Regulatory Agency (NPRA) Malaysia.
The departments included in the PRP training programme are regulatory affairs, pharmacovigilance, drug distribution, clinical research, medical affairs, sales & marketing, and other organized activities relevant to the industry. Other functions that may be covered are market access and trade marketing.
Regulatory affairs typically take up the longest duration of the one-year PRP training, covering various guidelines and processes such as application of import license, registration and maintenance of product license, review of marketing materials, application of clinical trial import license (CTIL), etc.
Medical affairs involve engagement with healthcare professionals, analysing scientific journals or articles, developing training materials for the salesforce, organizing webinars and advisory board meetings, to name a few. During the sales & marketing rotation, there would be opportunities to interact with healthcare professionals and other clients, attend Continuous Medical Education (CME) events, assist in developing promotional materials, and be exposed to brand planning and promotional strategy.
As clinical trials are vital in the drug development process, the attachment with Clinical Research Malaysia provides a good exposure to the processes and regulations involved in ensuring that each trial complies with the ICH Good Clinical Practice guideline and other relevant standards.
Pharmacovigilance is an important function as it ensures post-marketing surveillance of pharmaceutical products are carried out and reported to health authorities in a timely manner for further action if necessary.
These multinational pharmaceutical companies generally do not have their own warehouses for storage; an appointed distributor stores and distributes the pharmaceutical products to hospitals, clinics, pharmacies, etc. Control processes and workflows involved are covered in the attachment with the drug distributor.
For more information on learning objectives in the non-manufacturing pharmaceutical industry, click here to view the respective PRP logbook.

Manufacturing pharmaceutical industry:
While the PRP training in manufacturing pharmaceutical companies may not include some functions such as clinical research, pharmacovigilance, and medical affairs, PRPs in these companies will be exposed to various production processes and relevant guidelines such as product planning, compliance to Good Manufacturing Practice guidelines, facility and equipment management, core manufacturing processes, etc.
You will also be exposed to research & development of pharmaceutical products which includes understanding of various dosage forms, their development, bioequivalence testing for generics, method development and validation for new formulation. This would also be a good opportunity to learn about innovation in certain processes to increase productivity, efficiency or reduce cost, and also about filing patents for various formulations or procedures.
Hands-on training in the Pharmaceutical Quality Systems (PQS) will also be covered in the PRP training. These include understanding the concept of quality assurance (QA), key QA systems, and various qualification and validation requirements in the industry.
PRPs in the manufacturing pharmaceutical industry will be exposed to installation qualification, operational qualification, and performance qualification (IQ, OQ, PQ) which are important quality assurance protocols to ensure reliable performance of equipment used in the pharmaceutical industry. Warehousing and the supply chain system is part of the PRP training curriculum.
One of the ways regulatory affairs in the manufacturing pharmaceutical industry differ from the non-manufacturing pharmaceutical industry is from the aspect of product dossier preparation. This is usually done from scratch, whereas in multinational companies, product dossiers are prepared by international colleagues and sent to local regulatory affairs personnel to be submitted.
Though opportunities to interact with regional or international colleagues in multinational companies may seem more frequent than in local pharmaceutical companies, given that many of these companies are expanding to regional or even international markets, and driving an increase in export to other countries, it is likely that opportunities for international exposure may be within your grasp.
For more information on learning objectives in the manufacturing pharmaceutical industry, click here to view the respective PRP logbook.
2. What is the application process like?
The application process follows the typical job application methods i.e. sending emails to the preceptor in each company as listed in the Pharmaceutical Services Programme website to enquire on PRP vacancies, or applying through job sites such as JobStreet, LinkedIn, and Glassdoor.
LinkedIn is a good platform for connecting with people who are working in the companies you would like to apply to in order to find out if there are openings for PRP.
If you have successfully passed the screening stage, you will be invited for an interview. There may be two or three rounds of interview depending on the company.
3. What happens after the 1-year PRP programme?
Permanent placement in the company is not guaranteed after PRP training unless stated in the company offer letter. This is something which can be discussed with your preceptor before the end of your PRP-ship; alternatively, you can apply for vacant positions in the same company or in a different company.
4. Is it mandatory to do my PRP training in the pharmaceutical industry if I plan to work there in the future?
No, it is not compulsory to undergo PRP-ship in the pharmaceutical industry in order to secure a place in the sector. There are PRPs from the government sector and community pharmacies that have successfully transitioned into a career in the pharmaceutical industry.
Final Words
It is important to keep in mind that the objective of this PRP-ship is to learn and understand the nuances of the pharmaceutical industry in order to help you decide on your future path.
Be proactive in offering assistance to your colleagues, managers, customers etc. as there is always a learning opportunity. Don’t be afraid to make mistakes along the way and be accountable for them. Work smart, be flexible and be open to challenges.
Networking with colleagues or PRPs from other pharmaceutical companies are important, especially since you may need their assistance for job referrals in the future.
Every PRP will likely encounter different experiences during their apprenticeship. Seize the opportunity and make your own PRP experience a memorable and valuable one!

This article is contributed by Nicole Tay (former PRP in the non-manufacturing pharmaceutical industry) based on her experience as well as adapted information from the Pharmaceutical Services Programme website pertaining to PRPs in the pharmaceutical industry, and reviewed by Yong Wai Lee (former PRP in the non-manufacturing pharmaceutical industry) and Ooi Ziqian (former PRP in the manufacturing pharmaceutical industry). Individual experiences may vary depending on circumstances and pharmaceutical companies.
The opinions expressed in the article are the writer’s own and do not reflect the view of MPS YPC.
Cover photo: emerj.com

Nicole Tay is a regulatory affairs associate in the private sector. She graduated from International Medical University with BPharm (Hons) in 2019, and was formerly a PRP in the non-manufacturing pharmaceutical industry. Her interests include marketing, advancement in health technologies, and health policies.