New weed on the block?

Marijuana, also known as pot, weed and 420 among many other uniquely coded underground names is NOT the same as cannabis. More often than not, we tend to use them interchangeably perhaps due to the kind of western influence that we’re exposed to.
In actual fact, marijuana is merely a part of the product derived from the plant genus Cannabis sativa. The cannabis plant itself houses over 500 different chemical substances including marijuana. However, the crucial chemical substance found in the plant that’s all the rave at the moment is cannabinoids [1].
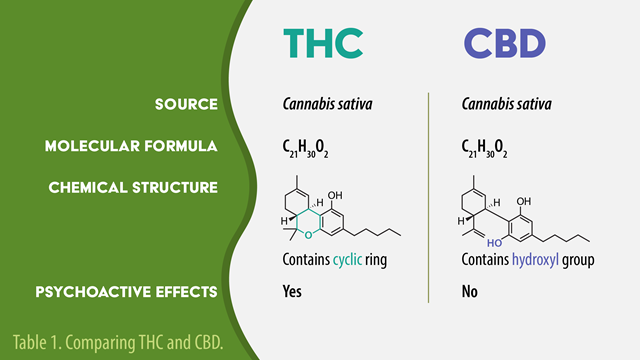
Cannabinoids can be branched out into 2 main groups, tetrahydrocannabinol (THC) & cannabidiol (CBD) with the former being commonly known for the psychological effect on moods or making a person ‘high’. On the flipside, CBD is non-intoxicating and does not impose psychoactive activity on those who consume it. Besides these two, there have been around 100 and 300 identified cannabinoids and non-cannabinoids respectively [2]. However, they both have similar chemical formulas consisting of 21 carbon atoms, 30 hydrogen atoms, and two oxygen atoms but are arranged differently. Thus, resulting in diverging chemical properties [3].
What’s all the storm about?
On the 14th of July, several major newspapers reported on the plan by the Ministry of Health (MoH) to introduce a framework for the registration of CBD products by 2023 [4]. Minister of Health, Khairy Jamaluddin mentioned how this is a step-by-step approach to legalise CBD in Malaysia. Claiming that it was the medical evidence related to CBD that drove forward this plan, he also mentioned that this framework is meant for CBD to be sold as prescription medicines and not for recreational or self-medication purposes.
CBD and its medical benefits
Cannabis sativa or the Indian hemp is a herbaceous plant originating from central and western Asia. Depending on the country, the plant can be cultivated for medicinal properties and hemp, a natural textile fibre [5].

Studies have reported plenty of pharmacological properties of the compound which ranges from being an anxiolytic, antiemetic, antipsychotic, anti-inflammatory, analgesic, antipsychotic and neuroprotective antioxidant [5]. There also have been many small scale studies conducted mainly in the US to provide evidence that CBD works therapeutically as it is claimed to be. For example, a study administering this compound to those who are heroin abusers found that within a week’s time, the CBD was able to reduce their withdrawal anxiety, resting heart rate and cue-induced cravings. Tapping into its anti-inflammatory and antioxidative properties, preclinical and clinical studies have hinted that CBD may be beneficial in neuroprotection especially in cases of Multiple Sclerosis, Alzheimer’s disease and Parkinson’s disease [6]. Particularly, there was a study done on effectiveness and safety of medical cannabis for treating cancer related symptoms in oncology patients in which it was found that significant improvements were seen in cancer comorbid symptoms within 6 months of treatment with cannabis. Although the changes in pain were relatively small, it was deemed that its use in cancer patients is safe and well tolerated [7].
Albeit all that, these studies are not catered to the local population within our country and even with such positive results, the small sample size used hinders its actual effect on a large population. Hence, the decision by the Health Minister to want researchers from Universiti Malaya to conduct clinical trials on the use of CBD for localized medical conditions is completely justified. The acknowledgement on use of cannabis for medicinal purposes was brought up last November and he mentioned that the existing legislations in Malaysia such as the Dangerous Drugs Act 192 and Sales of Drugs Act 1952 would be utilized to regulate cannabis and its by-products. [8].
The pharmaceutical scene
As of now, many countries globally have legalized the medical use of cannabis, including the likes of:
- Australia – regulated by Therapeutic Goods Administration (TGA)
- Brazil – regulated by Brazilian Health Regulatory Agency (ANVISA) under RDC No. 327 on 9 December 2019
- Canada – regulated by Health Canada under Marijuana for Medical Purposes Regulations
- Germany – regulated under German Narcotic Acts
- New Zealand – regulated under the Medicinal Cannabis Scheme with the commencement of the Misuse of Drugs (Medicinal Cannabis) Regulations 2019
- Switzerland – regulated under the amended Swiss Narcotics Act
- Thailand – regulated under the Marijuana and Hemp Bill
- the United Kingdom – governed by The Medical Cannabis (Access) Bill

Even in countries as massive as the US, the US FDA has not approved any marketing applications involving cannabis for the treatment of diseases or conditions. Those products that exist in their market are cannabis-derived or cannabis-related, both of which are only available upon prescription by licensed healthcare professionals. Having a pharmaceutical authority like the US FDA approves a cannabis-related product indicates that its safety and efficacy is well assured for the intended purpose. In the US, an oral solution is the first and only FDA-approved prescription cannabidiol used for treatment of seizures associated with tuberous sclerosis complex, Dravet syndrome or Lennox-Gastaut syndrome and in patients aged 1 year and older [9].
In 2014, the National Pharmaceutical Regulatory Agency (NPRA) approved an oromucosal spray called Sativex but was removed from the market three years later due to it not being commercially viable in the country. The spray consisting of cannabis extract with CBD and THC was used to treat muscle spasms and spasticity from multiple sclerosis. As of now, there are no existing cannabis products approved by the National Pharmaceutical Regulatory Agency (NPRA) in the Malaysian pharmaceutical market for any indication [8].
The future
With the ministry planning to release the framework for CBD products registration by next year, they have also invited proposals for registration of CBD products as well as the relevant authorities to provide education with regards to its prescribing and usage. On top of that, they are also looking to collaborate on clinical studies to research on the medical uses of marijuana which will no doubt benefit in building the framework and evaluating the necessity of having approved CBD products in the market [4].

It also helps in this endeavour that there have been reports that Duopharma Biotech Bhd, Malaysia’s largest pharmaceutical manufacturer is in the run to explore the probability of cannabis-related health care products in the market. Perhaps in the near future, approved prescription cannabinoids would also be available in Malaysia? Regardless, this is a huge step for both the pharmaceutical industry and MoH as it opens up a whole plethora of opportunities or possibilities. Hopefully it’s for the greater good but as with anything, it requires collaborative effort among MoH, NPRA and the pharmaceutical industry players in the country [8].
The opinions expressed in the article are the writer’s own and do not reflect the view of MPS YPC.
References
- Augustyn, A. ed., (2019). marijuana | History, Effects, THC, & Legality. [online] Encyclopædia Britannica. Available at: https://www.britannica.com/science/marijuana.
- Adf.org.au. (2022). Cannabinoids – Alcohol and Drug Foundation. [online] Available at: https://adf.org.au/drug-facts/cannabinoids/.
- DiLonardo, M.J. (2015). CBD vs. THC: What’s the Difference? [online] WebMD. Available at: https://www.webmd.com/pain-management/cbd-thc-difference#:~:text=lot%20of%20CBD.- [Accessed 24 Aug. 2022].
- Vethasalam, R. (2022). Khairy: Framework for registering Cannabidiol products to be released by next year. [online] The Star. Available at: https://www.thestar.com.my/news/nation/2022/07/14/khairy-cannabidiol-products-to-be-registered-next-year?fbclid=IwAR3nVXn-02OWLba2gQc8f2hfVwDuxztIj9j79p9SLN3e_dQIek4VBGb6VKw&fs=e&s=cl [Accessed 24 Aug. 2022].
- Meissner, H. and Cascella, M. (2020). Cannabidiol (CBD). [online] PubMed. Available at: https://www.ncbi.nlm.nih.gov/books/NBK556048/.
- Silva, L. and Gandhi, B. (2021). 9 Science-Backed Benefits Of CBD Oil. [online] Forbes Health. Available at: https://www.forbes.com/health/body/cbd-oil-benefits/.
- Aviram, J., Lewitus, G.M., Vysotski, Y., Amna, M.A., Ouryvaev, A., Procaccia, S., Cohen, I., Leibovici, A., Akria, L., Goncharov, D., Mativ, N., Kauffman, A., Shai, A., Bar-Sela, G. and Meiri, D. (2022). The Effectiveness and Safety of Medical Cannabis for Treating Cancer Related Symptoms in Oncology Patients. Frontiers in Pain Research, 3. doi:10.3389/fpain.2022.861037.
- Zainuddin, A. (2022). Malaysia Looks To Register Prescription CBD Drugs. [online] CodeBlue. Available at: https://codeblue.galencentre.org/2022/07/14/malaysia-looks-to-register-prescription-cbd-drugs/ [Accessed 24 Aug. 2022].
- FDA. (2021). FDA Regulation of Cannabis and Cannabis-Derived Products, Including Cannabidiol (CBD). [online] Available at: https://www.fda.gov/news-events/public-health-focus/fda-regulation-cannabis-and-cannabis-derived-products-including-cannabidiol-cbd#approved-.