Convalescent Plasma – Promising Rescuer of COVID-19?
I bet all of you have heard that COVID-19 has no approved specific treatment to date. Currently, clinicians are still counting on a few experimental agents which we have covered previously; Remdesivir, chloroquine and hydroxychloroquine. There’s a new kid on the block who’s creating quite a buzz recently; COVID-19 Convalescent Plasma. So, what is this therapy all about and the gazillion dollar question-is it effective?

Back to basics
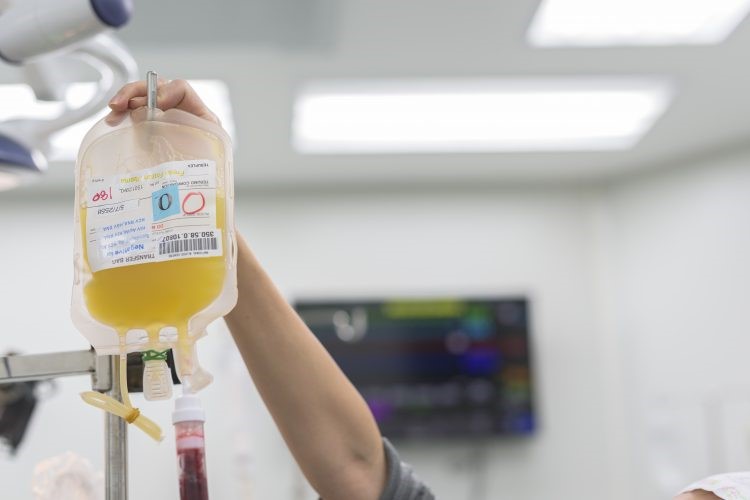
Convalescent plasma is the plasma taken from an individual that has recovered from an infection. Thanks to the hardworking, miraculous immune system, the recovered individual now has high level of antibodies in his/her plasma against the infectious pathogen. This antibody-rich plasma is then harvested and given to an infected patient to fight off the pathogen, leading to recovery and improved clinical outcomes. Immunity 101 refresher – basically Convalescent plasma therapy is another example of passive immunity besides breastfeeding and antisera which provide protection to the infected person without eliciting active immune response in his/her body.

Hey, I’ve heard that before!
That makes the concept of convalescent plasma therapy no stranger in the medicine world. It has previously been trialled in many viral diseases including Ebola, SARS, MERS-CoV and 2009 H1N1 flu either as prevention or treatment. Though some findings showed promising and beneficial results, the limitation of low quality studies made convalescent therapy reserved as the last resort if all other possible treatments fail or are unavailable.
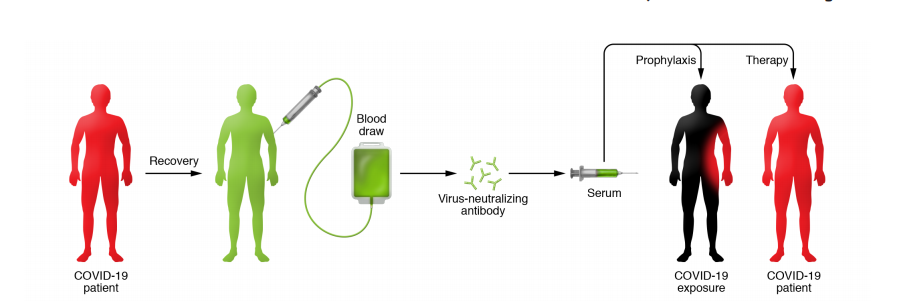
What about in COVID-19?
In COVID-19, the use of convalescent plasma is still at the experimental stage supported by two notable, low-quality articles. A pilot case control study conducted by Duan K et al (2020) and a case series by Shen C et al (2020) involving 10 and 5 severe COVID-19 patients respectively showed improvements in clinical symptoms. Although convalescent therapy showed potential therapeutic effect with low risk of adverse effect in severe COVID-19 patients, the limited sample size and study design rule out the effectiveness and safety of the therapy which require further evaluation from clinical trials. Based on historical experiences, administration of antibody can act as prophylaxis in high-risk population. However, its efficacy as prevention of COVID-19 is still unknown as no study on convalescent plasma as prophylaxis use has been conducted to date.
High hopes?
Hopes are high as clinical trials on the use of COVID-19 Convalescent plasma therapy have started in the United States and UK. Even some of our fellow Malaysians who have recovered from COVID-19 donated their plasma for this noble cause in the search of a cure. The US FDA has also provided guidance on collection and administration of COVID-19 Convalescent plasma as an investigational product. Via apheresis, COVID-19 Convalescent plasma is collected from donors who have recovered from COVID-19 for at least for 14 days and tested negative for COVID-19 or recovered for at least 28 days. The collected plasma with sufficient antibody titre against SARS-CoV-2 will be transfused into positive COVID-19 patients who are severe or at the ill-threatening stage. The COVID-19 Convalescent plasma can be obtained via clinical trial, expanded access or single patient emergency Investigational New Drug (IND) pathway under FDA. For further information on IND, visit the FDA’s website.
Not a rescuer (yet)!
With that being said, COVID-19 Convalescent plasma is still not approved for use. To-date, no specific prophylaxis and treatment is available for the treatment for COVID-19. Though promising, COVID-19 Convalescent plasma therapy requires further investigations to assess optimal dosage, treatment time point, safety and efficacy which is also in line with recommendations from Ministry of Health, Malaysia. Evidence on COVID-19 is ever-evolving so let’s be optimistic on the ongoing clinical trials of COVID-19 Convalescent plasma therapy. In the meantime, all we can do is soldier on and fight COVID-19 together. #staysafe

The opinions expressed in the article are the writer’s own and do not reflect the view of MPS YPC.