Pharmacists and COVID-19 Vaccination – What do we do?

The world has been affected with the COVID-19 pandemic since Dec 2019 and one year later, a knight, or perhaps knights in shining armors – COVID-19 Vaccines have come to the rescue! In Malaysia, COVID-19 Vaccines have started to be rolled out since 24th February 2021 with our PM and Health DG being the first ones to receive the vaccine respectively.
It is well-known by the general public that vaccination is often linked with doctors and nurses in our local healthcare scene but in this article, we will shed some light on the roles of pharmacists in this massive National COVID-19 Vaccination program.
1. Regulatory
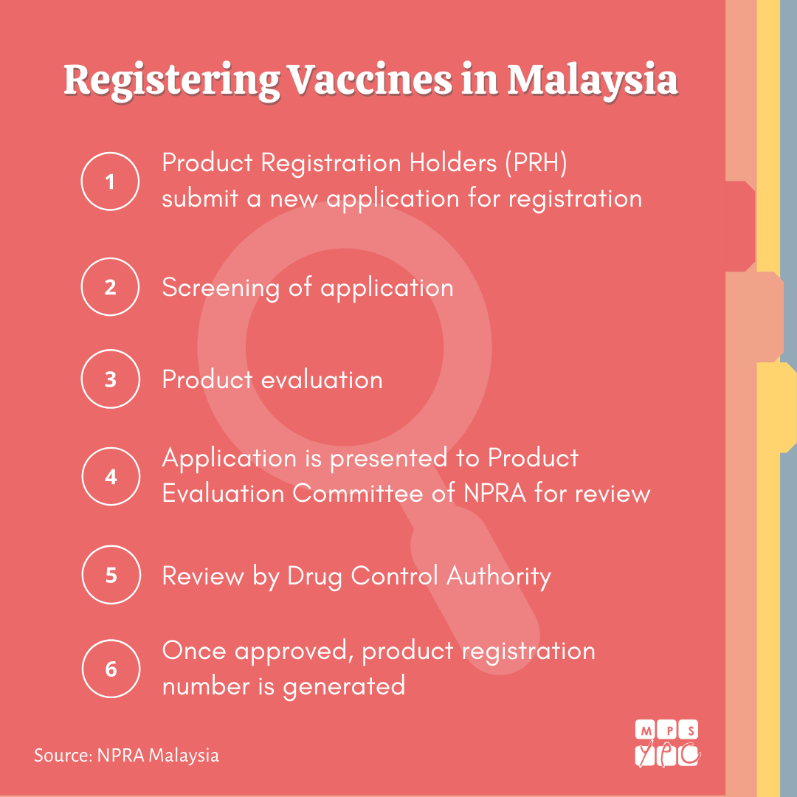
Pharmacists in National Pharmaceutical Regulatory Agency (NPRA), the Malaysian version of FDA are one of the first-line inspectors before the vaccines are accessed by other parties. NPRA, under the Ministry of Health, is responsible in safeguarding the nation’s health through scientific excellence in strict regulatory control of medicinal products and cosmetics. Before we can start using the vaccines, they need to be registered and approved. NPRA will evaluate dossiers (registration application data) from the manufacturers and other related data (e.g. stability data of finished product from packaging plant) to ensure validity, safety and efficacy of these vaccines before they are reviewed and approved by the Drug Control Authority. Under the law (Control of Drugs and Cosmetic Regulations 1984), only registered pharmaceutical products can be manufactured, sold, supplied, imported, possessed or administered in Malaysia.
Retrieved from MPS-YPC FB post on How Vaccines are Regulated in Malaysia
2. Procurement, Distribution, Receipt and Handling COVID-19 Vaccines
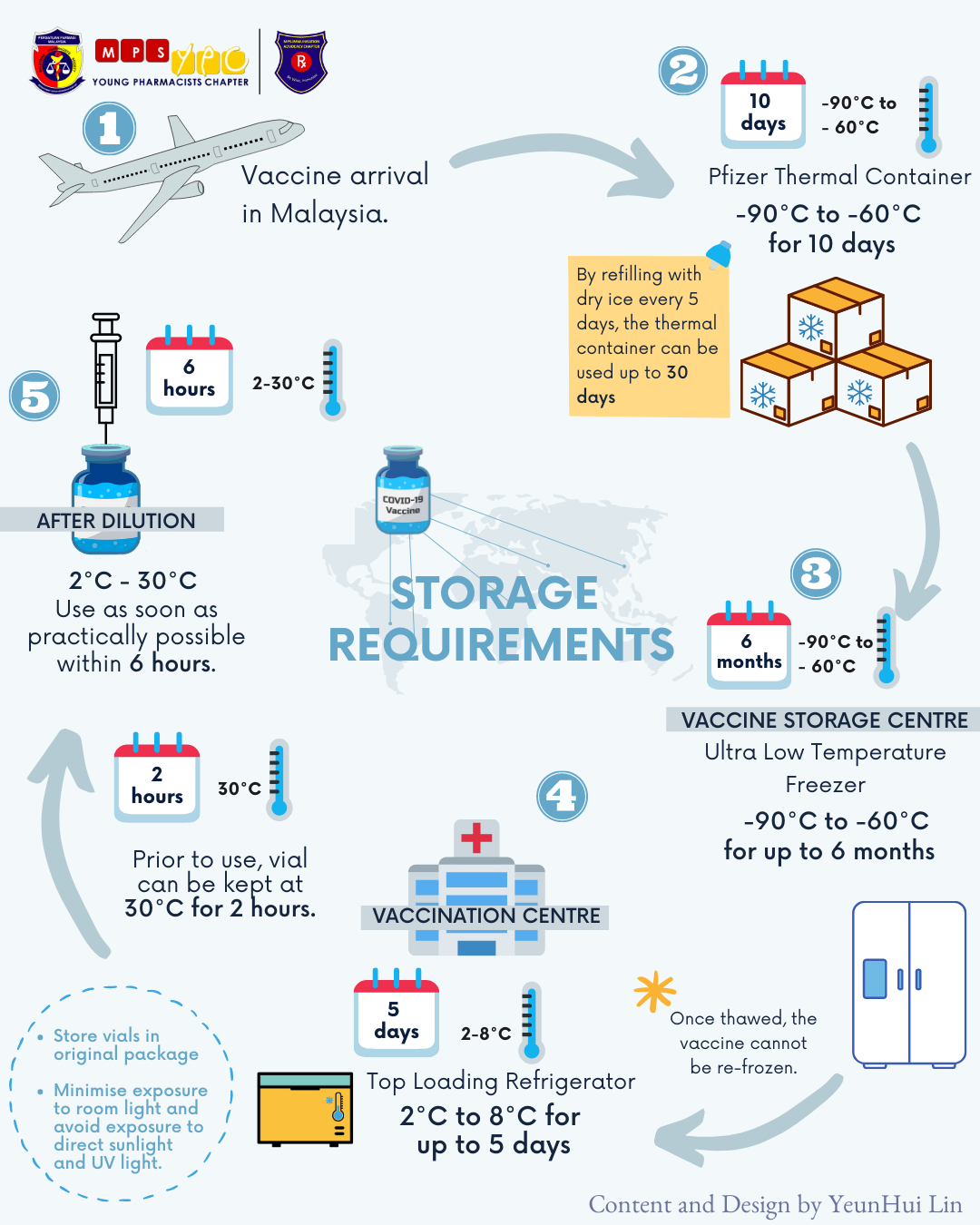
Once vaccines are approved by the NPRA, we can start anticipating the arrival of these precious gems. It is definitely challenging to adhere to the storage requirements of COVID-19 Vaccines particularly the COMIRNATY vaccine from Pfizer-BioNTech. The strict condition to store the vaccines at ultra-low temperature of -90 to -60°C (Comirnaty) or 2-8°C are necessary to ensure the efficacy and stability of the vaccine. Regular temperature monitoring needs to be conducted to ensure the temperature of freezer and/or refrigerator is within the allowable range. Even during vaccine transportation from the Vaccine Storage Site to Vaccination Site, these vaccine temperatures are required to be maintained within the range.
Besides that, pharmacists are also in charge of allocating and distributing correct amount of vaccine vials from the Vaccine Storage Site to tally with the expected vaccine doses to be given for that day or week. This is to prevent wastage as thawed vaccine vials cannot be re-frozen thus cannot be used again as the stability of the vaccine may have been affected. Imagine how melted ice-cream cakes lose its shapes when refrozen. In terms of a vaccine, its ingredients’ properties might be altered thus may no longer work as effective.

Storage Requirements of COMIRNATY Vaccine (Retrieved from MPS-YPC FB Post)
3. Monitoring and reporting Adverse Event Following Immunization (AEFI)
No pharmaceutical product in the world comes without side effects, and there are no exceptions for the COVID-19 Vaccine. Adding to the fact that the COVID-19 vaccine is newly registered, it is of utmost importance to report any adverse events that happen after vaccination. This is to ensure that unwanted effects are detected by the drug monitoring system. This is because rare and very rare adverse events may occur when a much larger population with diverse backgrounds are receiving the vaccine compared to the trial population.
In public healthcare settings, pharmacists are in-charge of monitoring and reporting AEFI. They are part of the investigation team for serious AEFI and are tasked with the role of liaising with NPRA for complete data collection for analysis. These data are crucial to monitor and compare with background rate that could identify either product quality defect, immunization error or increased susceptibility of vaccine reaction among the given population.
Community pharmacists can also assist their customers/patients in the community to report AEFI given that community pharmacies are easily accessible. Reporting AEFI can be done by community pharmacists through NPRA website. This can help to improve data collecting up to community level to detect possible safety issues on COVID-19 vaccines.
Different ways to report AEFI as a Healthcare Professional (Retrieved from MPS-YPC post)
4. Advocate for COVID-19 Vaccination
Armed with knowledge pertaining to medications and being easily accessible, pharmacists can provide correct information about COVID-19 vaccines to the general public. Vaccine hesitancy has been one of the major global health threats even before COVID-19 came knocking on our doors and it will continue to be a huge issue to tackle as we aim to educate the majority population to be vaccinated with COVID-19 vaccine. As healthcare professionals, it is important to keep ourselves informed and updated with the latest news and ever-changing evidences about COVID-19 and the vaccines to ensure the information that we spread are always accurate and reassuring. Click here to keep yourself posted on some of COVID-19 vaccine infographics produced by our own YPC Publicity Team.
5. Pharmacists at Vaccination Centers
Some pharmacists are deployed to vaccination centers to be in-charged of the vaccines — from collecting vaccine supply from vaccine storage sites to ensuring vaccines are stored and administered appropriately in the vaccination centers so that each vaccine vial is put into good use and tallied to avoid wastage. Besides, pharmacists also assist doctors in screening vaccinees prior to obtaining consent for vaccination on-site. Information on vaccinees’ previous & current medical and medication and allergy history, pregnancy & breastfeeding status (for women) are acquired and analyzed for risk assessment to receive the COVID-19 vaccine. Pharmacists, along with dentists, are also tasked with the crucial role of giving the green light for for vaccinations. Answering enquiries from the public and briefing vacinees on the expected side effects that require immediate medical attention are also provided by pharmacists at these centers to educate and reassure the public.
6. Administering vaccines
Last but not least, administering vaccines. This may be a novel thing for pharmacists in Malaysia but it has been one of the roles of pharmacists in 36 other countries such as the United States, UK and Australia to name a few. The Malaysian Pharmacists Society has proposed to our government to include community pharmacists as immunisers who can administer COVID-19 vaccines to the public. Pharmacies are omnipresent and are thus ideal vaccination sites. With proper recognition, investment and training, pharmacists can play a more significant role to achieve high vaccination coverage and herd immunity as soon as possible.
Healthcare is indeed multi-disciplinary and all professions involved, including pharmacists, are vital in ensuring that the nation is as healthy as possible. To pharmacists who have yet to take a breather since the first case of COVID-19 was reported, this article is dedicated to you. The hard work, dedication, and perseverance of you front liners are what’s keeping this machine which is the healthcare running. Hat’s off to you, my fellow comrades. Take a bow.